Labels are the centerpiece of almost every product’s packaging. They act as in-store sales pitches to potential consumers and represent your brand. Every business strives to design attractive and memorable product labels - but there’s more to labels than just looks. Federal law places strict requirements for information that must be included on food and beverage labels. The published rulebooks can be difficult to understand, but making a mistake with label information can result in your product being pulled from shelves. This guide will help you design a legally compliant and informative label, so you can keep selling your product.
Food and Drug Administration Requirements
The Food and Drug Administration (FDA) is a federal agency that operates as part of the U.S. Department of Health and Human Services. It’s mission is to protect the public health by assuring the safety, effectiveness, quality, and security of a myriad of products, including food, beverages, and cosmetics. The FDA has rigid requirements for the information that must be included on food and beverage labels, and also regulates the manner in which this information can be presented on a label. Here's a rundown of the FDA's requirements on several parts of your label:
Statement of Identity
First thing's first - your label needs to include name of your product. According to the FDA, the name should be the established and commonly used title of a product. Although, if the nature of the product is obvious, you have the option to use a more elaborate name. Your design should include the identity statement in prominent typeface, and should place it on both the front label and any other principal display panels (PDPs). It must be positioned generally parallel to the base of the package, and should appear at least half the size of the largest print on the label.
Net Quantity of Contents
The net quantity statement declares the total amount of food in the package. For liquid products, express net quantity in liquid measure (fluid oz., liters). For other products, you can list net quantity in either weight, numeric count, or measure. Make sure you write the quantity in both U.S. Customary and the metric system.
Your design should include the net quantity statement located in the bottom 30% of the front label as a distinct item parallel to the package’s base. As you select a print style, ensure it is prominent and easy to read. It’s sizing should adhere to the following requirements:
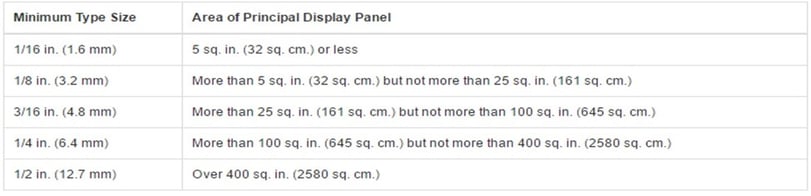
Image Source: U.S. Food & Drug Administration
Manufacturer’s Information
The label must contain the name and address of the manufacturer, packer, or distributor. The address should always consist of city or town, state and ZIP code. Only include the street address if the firm name and address are not listed in a current city directory. If you are printing a packer or distributor, you also need a phrase that states the firm’s relation to the product. You have freedom to choose any typeface and location for the statement.
Nutrition Facts Label
Label Design
The nutrition facts label provides the most important information on your entire label. You should always design the nutrition label in a box to set it apart from other information. You can group this label with the address and ingredient list on the PDP, the panel adjacent to the right of the PDP, or on the next adjacent panel to the right.
Your nutrition label can feature any legible type style, but must include the title “Nutrition Facts” spanning the width of the box. You must make this title stand out by printing it in the largest type-size in the nutrition label. You have freedom to alter the thickness of the three bars separating the central portions of the label. Below is a sample standard Nutrition Facts label from the FDA guidelines.
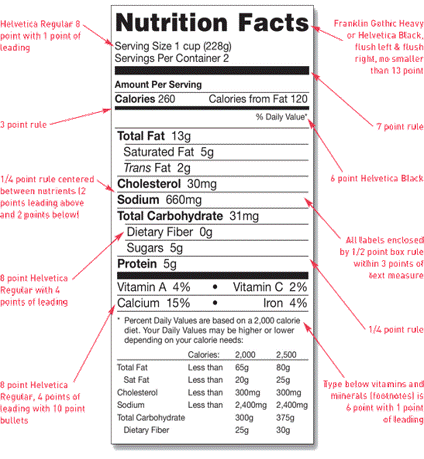
Image Source: U.S. Food & Drug Administration
Label Content
Determine the serving size of your product and place this above the first horizontal bar in the box. If your product is multi serving, you should list the serving size using household measure, followed by metric measurement in parentheses. If you have a single serving product, you can write the serving size as “1 package”, “1 cup,” et cetera.
To determine serving sizes, use the two tables in Section 101.12(b) of the food labeling regulations to find the Reference Amount Customarily Consumed (RACC) for your product. You can base all following nutrition facts on this serving size.
Next, you should include the caloric content of your product, and list the calories derived from fat. Below this, you need to list the amount, in grams, of the five major nutrients: fat, cholesterol, sodium, carbohydrates, and protein.
You should also list the percent daily values of each of these nutrients. In your calculations, use the total daily values of each nutrient listed here. Follow these nutrients with a horizontal bar, then list the percent daily values of vitamins and minerals.
Ingredient Statement
On most labels, ingredient lists directly follow nutrition labels. You must place this list on the same panel as the name and address of the manufacturer/distributor/packer. For this label component, you should list every ingredient, in descending order of weight. You should pick a typeface that is legible and conspicuous (for measurement purposes, the FDA requires that the lowercase “o” letter is at least one sixteenth of an inch tall).
Your ingredient list should be immediately followed by an allergen warning if your product contains any of the following eight foods or proteins derived from these foods: milk, egg, fish, crustacean shellfish, tree nuts, wheat, peanuts, or soybeans. The warning should consist of the word “Contains” followed by a colon and the included allergen foods.
/iStock-577950664-min.jpg?width=811&height=540&name=iStock-577950664-min.jpg)
Genetically Modified Organism Labeling Laws
FDA regulated food labeling laws have been established for at least two decades, however, brand new federal legislation will soon be affecting your product label design. The federal government recently passed the National Bioengineered Food Disclosure Standard as an amendment to the Agricultural Marketing Act of 1946. The bill regulates label declarations of food and beverage products that contain GMOs. Although these new regulations will not go into effect until the summer of 2018, you should design product labels to include them now.
If your food or beverage product contains genetically modified ingredients, it will be mandatory for your label to include one of the following:
- A QR code: Consumers will scan this code with smartphones, and land on a webpage that should contain information regarding GMO content.
- A 1-800 phone number: Consumers have the option to call this number for information regarding the presence of GMOs in your product.
- A symbol: The symbol has yet to be designed, but will represent the presence of GMOs. Consumers will not need to take any steps to find out information about GMO content.
- A designated phrase: The phrase has not been designated yet, nor have any text size or location requirements.
Although the details of these requirements are not fully defined, you should visualize how to incorporate one of the options into your label design. Make sure you stay up to date as the regulations are finalized, so you will be ready for the 2018 kickoff of the law.

/iStock-577950664-min.jpg?width=770&height=404&name=iStock-577950664-min.jpg)


/iStock-577950664-min.jpg?width=811&height=540&name=iStock-577950664-min.jpg)
.png?width=480&height=252&name=PRESS%20RELEASE-2%20(4).png)

